9月3日,默克将在上海办公室举办首届开放创新日(Open Innovation Day),与合作伙伴共同探索创新治疗药物,以满足我们专注治疗领域中未被满足的临床需求。
我们诚邀创新者向默克医药健康全球领导者展示其开创性的科学成果,与行业领袖交流并深入了解治疗前沿。活动将以闭门形式进行。
我们关注的方向
在以下领域的创新资产、模式和技术:
心血管、代谢与内分泌
生殖
神经学
肿瘤学(重点关注实体瘤)
以人工智能(AI)和机器学习(ML)为核心,旨在提升药物发现与开发的方案。
此外,我们寻求在构思、原型设计、试点和商业推广各阶段具有实质性创新的项目,这些项目应符合默克关注的重点领域,并具备清晰的商业化路径。我们鼓励建立在切实可行、可扩展的解决方案基础上的早期创新。本次活动不设现金奖励。
我们的评估标准
模式和技术的创新性
· 在疗效、安全性、给药途径等方面显著优于现有标准疗法和在研资产,为患者提供更优治疗获益。
· 创新性需有扎实数据,或临床试验结果支持。
治疗领域的相关性
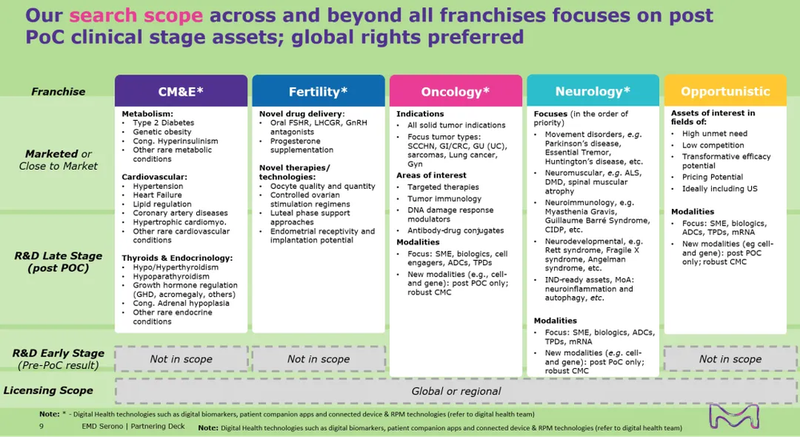
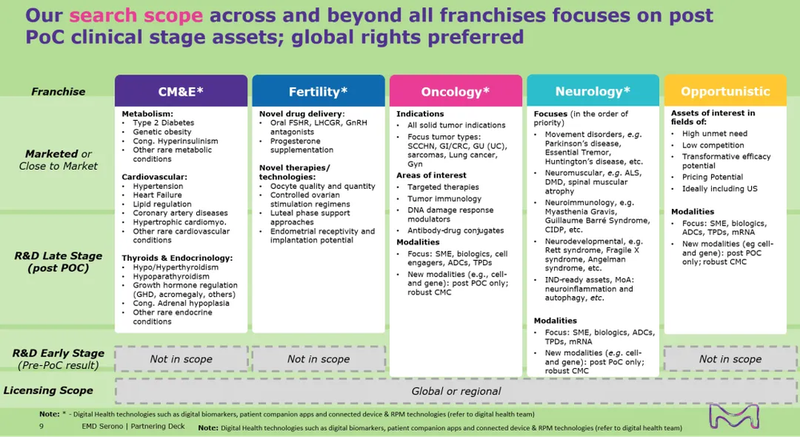
· 与默克的重点关注领域保持一致,包括心血管、代谢与内分泌、生殖、神经学、肿瘤学(重点关注实体瘤)和相关技术平台。
临床验证水平
· 心血管、代谢与内分泌和生殖领域:希望具有临床概念验证(PoC),我们将优先考虑处于III期及以后的资产。
· 神经学和肿瘤:我们对各个阶段的资产持开放态度,强有力的PoC将是加分项。
· 技术平台:我们倾向于已有临床应用的案例。
推介方案的完整性
我们希望提案中包含以下内容:
公司简介
· 历史、业务重点、地理位置、规模、财务状况及管理团队信息。
技术和产品管线
· 核心技术平台的科学原理及应用详细描述。
· 管线概览,包括项目名称、作用机理(MoA)、适应症、开发阶段以及权利持有者或合作伙伴信息。
资产详情
· 疾病背景:流行病学数据、当前标准护理、未满足的需求及市场潜力;
· 作用机理(MoA)与靶点理论;
· 差异化策略及支持数据;
· 目标产品概况(TPP),尤其针对后期产品。
· 竞争格局:与标准护理和新兴竞争者的简要对比(涵盖作用机理、给药途径、目标患者人群、治疗方案,以及临床前和临床数据的疗效与安全性对比)。
· 临床开发计划及注册路线:已完成、正在进行及计划中的试验,关键的监管里程碑(请采用甘特图展示的时间表)。
· 代表性结果:研究设计、数据及结论,并提供交叉试验比较的数据来源引用。
· 与药品监管机构的沟通结果。
· CMC及材料供应情况,尤其针对后期资产和先进技术领域。
· 知识产权信息:包含任何上游义务。
· 合作潜力及双方的战略契合度。
· 首选合作模式及期望,例如对未来临床试验的投资及保留的权利。
· 联系方式。
为符合保密要求
包括主要亮点的基础上可以简要陈述
团队的资质和能力
请重点强调团队的能力
申请安排与方式
申请提交时间:7月至8月初
为确保流程迅速响应,申请将采取“滚动评审制”
符合条件的申请者将获邀进行展示
请将包含非保密信息的推介方案及
相关支持文件发送至:grace.xiao@merckgroup.com
我们鼓励所有致力于推动行业进步的创新者的积极申请,把握这一在该领域产生深远影响的机会,共同推动医药健康领域的创新与合作!
Merck is excited to announce the Merck Open Innovation Day in China, aimed at identifying the next generation of therapeutics that address the unmet needs in our focused therapeutic areas. This event invites innovators to showcase their groundbreaking science to Merck Healthcare leaders in a unique closed-door presentation format.
Scheduled for September 3, 2025, at the Merck office in Shanghai, this initiative offers an excellent opportunity for innovators to connect with industry leaders and gain valuable insights into the therapeutic landscape. We will be accepting applications from July to early August. To ensure a swift and efficient process, applications will be reviewed on a rolling basis, with invitations extended to pitch and present.
We encourage all innovators passionate about advancing healthcare to apply and seize this opportunity to make a significant impact in the field. Join us in our mission to foster innovation and collaboration in the healthcare sector!
What we're looking for
Innovators are invited to submit their novel assets, modalities and technologies that demonstrate superior safety and efficacy in Cardiovascular Disease, Metabolic Disease and Endocrinology, Fertility, Neurology, and Oncology with focus on Solid Tumors. Proposals focused on AI and machine learning to enhance drug discovery and development are also welcome.
We seek tangible innovations across all stages of development—from ideation and prototyping to piloting and commercial launches. Submissions must align with Merck's strategic priorities in our focus areas and demonstrate a clear path to commercialization. While early-stage ideas are encouraged, proposals should be grounded in practical, scalable solutions rather than purely academic research.
Please note that no monetary award will be offered for this challenge. Instead, this presents a valuable opportunity to showcase your innovation to Merck Healthcare leaders, to receive their insightful feedback and to network with leading industry peers.
Specific novel modalities and technologies include
Next generation Antibody-Drug Conjugates
Cell Engagers
Nucleic acid therapies, such as small interference RNA (siRNA) Therapies and circular RNA
Oral Peptides or proteins
Treatment of male/female infertility, endometriosis, PCOS, ovarian insufficiency, novel delivery route of sex hormone, and AI to improve patient journey
Platforms to identify novel antigens or to generate differentiated tumor-specific binders
Criteria
Potential applications will be assessed by a panel of reviewers based on their ability to meet the following criteria:
Level of innovativeness of the asset, modality or technology
The opportunity should be highly innovative and differentiated to Standard of Care and in-development assets in terms of efficacy, safety, route of administration, and other factors, offering significantly better clinical benefit to patients. The innovativeness must be supported with solid data, ideally clinical results.
Strategic alignment or relevance to Merck's focused therapeutic areas
The submitted opportunity should be relevant to Merck's search scope (see Appendix A), which includes Cardiovascular Disease, Metabolic Disease and Endocrinology (CM&E), Neurology, and Oncology, with focus on Solid Tumors.
Validation level and/or proof of concept
For CM&E and Fertility, clinical proof-of-concept (POC) is preferred, and assets in Phase 3 or later stages will be prioritized. For Neurology and Oncology, we are open to considering assets at all stages, with a strong clinical POC being a plus. For technology platforms, validation through use cases in the clinical stage is preferred. Please note that a high level of innovativeness is always required.
Comprehensiveness of pitch deck
We expect the elements listed in Appendix B to be included in the proposal. A business-friendly PowerPoint format is required. The description can be brief at the proposal submission stage to accommodate non-confidential requirements, but it should be sufficient for reviewers to identify the key highlights.
Quality and capabilities of the team
Please highlight the team's capabilities, particularly those of key individuals who provide the company with unique strengths
Please submit the pitch deck containing non-confidential information along with any supporting documents to the following address: grace.xiao@merckgroup.com
Appendix A: Merck Search Scope
Appendix B: Key Elements in Pitch Deck
Company profile:History, business focus, location, size, financial health, and management team.
Technology and pipeline: Scientific rationale and applications of the core technology platform, along with a snapshot of the pipeline that includes program code, mechanism of action (MoA), indication, development stage, and rights holder/collaborator.
Asset profile
· Disease background, including epidemiology data, current SoC, unmet needs, and market potential
· Mechanism of action (MoA) and target rationale
· Differentiation strategy with supporting data
· Target product profile (TPP), especially for late-stage assets
· Competition landscape: A concise comparison to SoC and emerging players, including MoA, route of administration (RoA), patient segment, treatment regimen, and non-clinical and clinical efficacy and safety data
· Clinical development plan and roadmap towards registration, including completed/ongoing/planned trials, along with regulatory milestones, presented in a detailed list and a Gantt chart to show the timeline
· Representative results including study design, data, and conclusion, with citations for data sources for cross-trial comparison.
· Outcome of communications with health authority (HA)
· CMC and material supply, especially for late-stage assets and advanced technology areas
· IP information, including any upstream obligations
· Collaboration potential and strategic fit between both sides
· referred collaboration model and expectations, such as investment in future clinical trials and retained rights.
· Contact information

向下滑动查看更多
