2024年FDA批准新药汇总
2024年10月12日
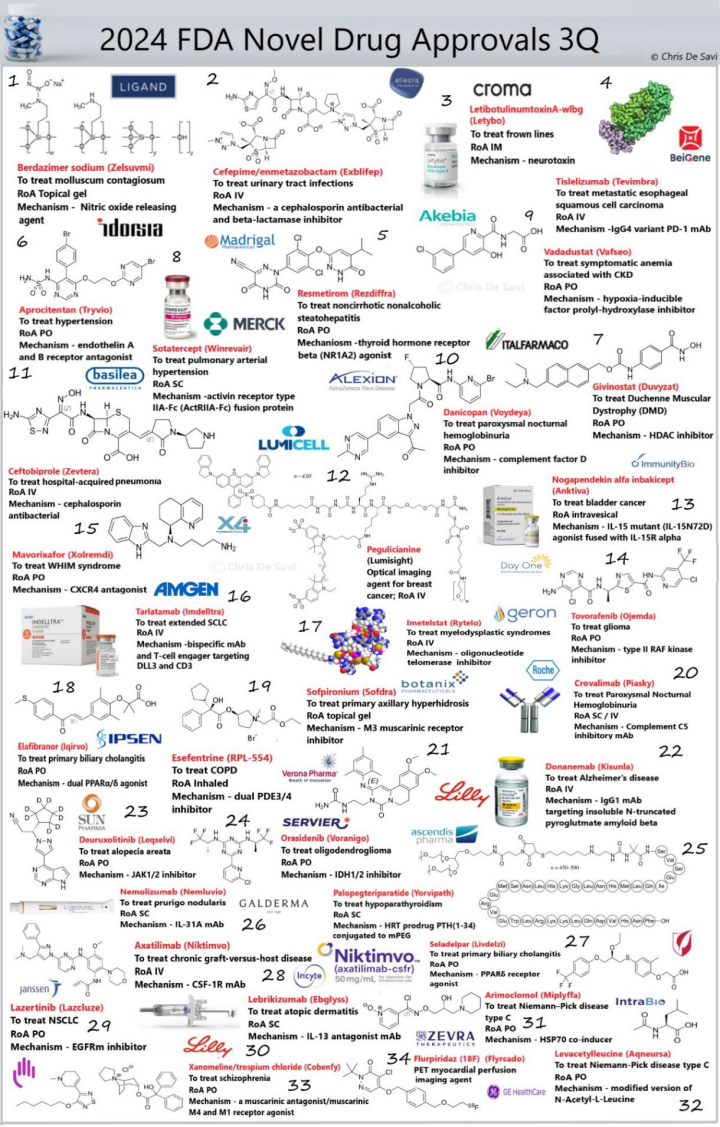
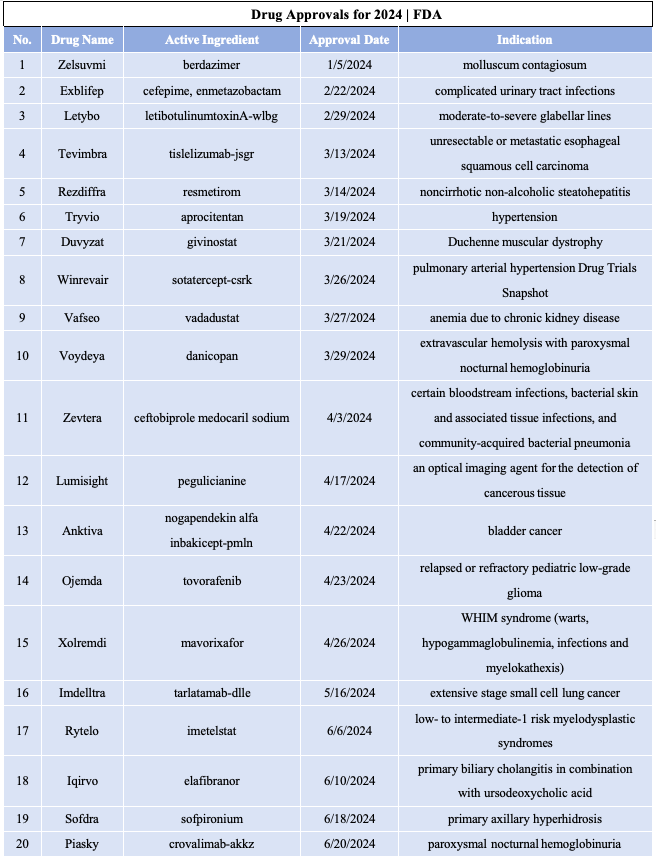
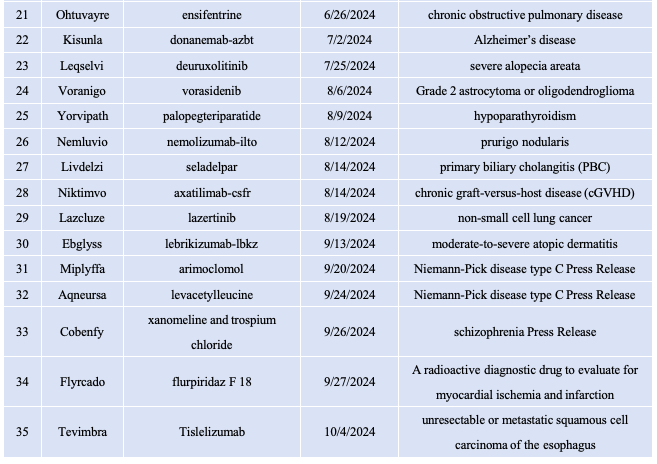
截止目前,FDA已经批准35款新药上市,其中多款药物具有里程碑意义。 1.1.Zelsuvmi (berdazimer)
1.2.Exblifep (cefepime and enmetazobactam)
企业:Orchid Chemicals & Pharmaceuticals Ltd.
1.3.Letybo (letibotulinumtoxin a-WLBG)
1.4.Tevimbra (tislelizumab-JSGR)
1.5.Rezdiffra (resmetirom)
1.8.Winrevair (sotatercept-CSRK)
企业:Achillion Pharmaceuticals Inc.
1.11.Zevtera (ceftobiprole medocaril sodium)
适应症:血液感染、细菌性皮肤和相关组织感染、社区获得性细菌性肺炎
1.13.Anktiva (nogapendekin alfa inbakicept-pmln)
企业:Altor Bioscience Corp.
1.14.Ojemda (tovorafenib)
企业:Viracta Therapeutics Inc.
1.15.Xolremdi (mavorixafor)
1.16.Imdelltra (tarlatamab-dlle)
1.18.Iqirvo (elafibranor)
1.19.Sofdra (sofpironium)
企业:Bodor Laboratories Inc.
1.20.Piasky (crovalimab-akkz)
企业:Sino-Foreign Pharmaceutical Co., Ltd.
1.21.Ohtuvayre(Ensifentrine)
1.22.Kisunla(Donanemab-azbt)
1.23.Leqselvi(Deuruxolitinib)
1.24.Voranigo(vorasidenib)
适应症:IDH1或IDH2易感突变的2级星形细胞瘤或少突胶质细胞瘤。
企业:Servier Pharmaceuticals
1.25.Yorvipath(palopegteriparatide)
1.26.Nemluvio(nemolizumab-ilto)
1.27. Livdelzi(seladelpar)
1.28.Niktimvo(axatilimab-csfr)
1.29.Lazcluze(lazertinib)
1.30.Ebglyss(lebrikizumab-lbkz)
1.31.Miplyffa(arimoclomol)
1.32.Aqneursa (levacetylleucine)
1.33.Cobenfy(xanomeline and trospium chloride)
1.34.Flyrcado(flurpiridaz F 18)
适应症:评估心脏血流阻塞(心肌缺血)和心脏病发作(心肌梗塞)
1.35.Tevimbra(Tislelizumab)
Voranigo(vorasidenib)这种双重IDH1/IDH2抑制剂处理导致癌细胞代谢变化的突变。它被批准用于2级星形细胞瘤或少突胶质细胞瘤,这是一种生长缓慢的脑肿瘤,具有显著的风险。这是一个突破,因为它直接针对驱动肿瘤生长的突变,提供了一种更有效的治疗选择,可以延缓疾病进展,减少对辐射等侵入性治疗的依赖。
Niktimvo(Axatilimab)是一种CSF-1R单克隆抗体,通过限制巨噬细胞活性来治疗cGVHD,巨噬细胞在免疫应答中起着至关重要的作用。这种疗法对患有cGVHD的患者至关重要,这是干细胞移植后的一种严重并发症,通常对现有治疗无效。它提供了一种更有针对性的策略来改善患者的生活质量。
Cobenfy作为Xanomeline和Trospium Chloride这种联合治疗方法,通过调节毒蕈碱受体,为治疗精神分裂症带来了新的途径。通过平衡大脑中的神经递质活动,它解决了这种疾病的阳性和阴性症状,这使得它对那些用其他药物都没有成功的患者特别有价值。它的双重机制提供了更全面的症状管理,改善了那些受这种具有挑战性的精神健康状况影响的结果。
Rezdiffra(resmetirom)是一款甲状腺激素受体(THR)-β口服选择性激动剂,THR-β在肝脏中高度表达,能够调节脂代谢,降低LDL-C、甘油三酯和致动脉粥样硬化性脂蛋白。此外,THR-β还能通过促进脂肪酸的分解和刺激线粒体的生物发生来减少脂肪毒性并改善肝功能,从而减少肝脏脂肪。Rezdiffra用于治疗患有中重度肝纤维化(F2至F3期)的非肝硬化非酒精性脂肪性肝炎(NASH)患者,成为FDA批准的首款NASH疗法,具有里程碑意义。
Anktiva是一款IL-15超级激动剂。IL-15通过影响自然杀伤(NK)细胞和免疫T细胞的发育、维持和功能,在免疫系统中发挥着至关重要的作用。Anktiva由与IL-15受体α/IgG1 Fc融合蛋白结合的IL-15突变体(IL-15N72D)组成。它与βγ T细胞受体结合,直接特异性刺激CD8阳性T细胞和NK细胞,同时避免刺激调节性T细胞(Treg)。与天然、非复合IL-15相比,Anktiva在患者体内具有更优的药代动力学特性,且能在淋巴组织中存在更长时间,表现出增强的抗肿瘤活性。Anktiva在2019年获FDA授予突破性疗法认定,用于与BCG联合治疗此前对BCG应答不佳的非肌层浸润性原位膀胱癌患者。参考资料
[1]Novel Drug Approvals for 2024 | FDA
[2]百济神州官网